PLEASE NOTE: A more technical version of this article, intended for professionals in the field, can be found at this link. In addition, these are both works in progress with additional text, videos and images to be added as appropriate. Is that sensational scientific study really so important that you have to change your life? […]
Archive | Featured

A Strength Of Evidence Report Card For Human Studies
PLEASE NOTE: A less technical version of this article, intended for non-scientists, can be found at this link. Beta Version 4.7 — 03/06/24. This strength-of-evidence report card was developed by Lewis Perdue and will be updated and/or expanded as needed. It is based upon the concept of evidence-based medicine which was developed to help clinicians […]
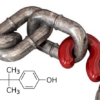
Double-blind, randomized, controlled human clinical trial study to explore the causal relationship between the measured dosed exposure of three common bisphenols, and a panel of metabolic and inflammatory blood markers.
This post written by Study Designer Lewis Perdue. Following is the text summary of a study developed by Co-Principal Investigator Lewis Perdue to be co-conducted with Victor Reus, M.D., The Distingushed Professor of Medicine at UCSF. The study is currently being evaluated by the University of California San Francisco School of Medicine, Institutional Review Board. […]

Playing Whack-A-Mole With Plastics And Your Health
This is a true tale for modern consumers, based upon results from a scientific paper created by the Center for Research on Environmental Chemicals In Humans. In science, as well as in classic mysteries, that which is missing often provides a pivotal clue discoverable only after a bit of detective work. In Sir Arthur Conan […]

How Dairy Products Complicated Our 2022 Study – And What We Had To Do To Solve The Issue
When we conducted the investigation for our 2022published paper, the goal was to create a menu that was a “typical” American diet as defined by the U.S. Department of Agriculture. That “typical” diet demanded that we include cow’s milk. And that was a problem. Dairy has many opportunities for plastic contamination For dairy products, we […]

High Profile Dietary Intervention Fails to Approach Causality and Replicability
NOTE: An earlier, May 2020 look at this issue can be found here: Significant changes necessary in order for dietary intervention studies to be causal and replicable This ad-free article is made possible by the financial support of the Center for Research on Environmental Chemicals in Humans: a 501(c)(3) non-profit. Please consider making a tax-deductible donation for continued […]
Published Trial Results Indicate That All Dietary Interventions So Far Are Not Replicable. New Protocols Suggested to Assure Reproducible, Useful Results
The text as published at medRxiv is at this link: Investigating hsCRP as a clinical inflammation marker for human Bisphenol A food contamination offers protocol suggestions for conducting replicable, causal dietary intervention studies Investigating hsCRP as a clinical inflammation marker for human Bisphenol A food contamination offers protocol suggestions for conducting replicable, causal dietary intervention […]
Moving Toward the Trial: Some Final Thoughts on Procedures, Methods, Food Acquisition, and Best Practices
Developing a scientifically sound set of methods for a dietary intervention that could result in replicable, causally valid results took investigators five years of intensive effort. That was a necessary first step because no such standards, protocols, methods or best practices existed. That absence required investigators to develop those standards and protocols de novo in […]

Significant Changes Necessary in Order for Dietary Intervention Studies to be Causal and Replicable
Dietary intervention studies as a whole are inherently flawed and do not produce causal or clinically relevant health recommendations or decisions. This ad-free article is made possible by the financial support of the Center for Research on Environmental Chemicals in Humans: a 501(c)(3) non-profit. Please consider making a tax-deductible donation for continued biomedical research. Accuracy and replicability of […]