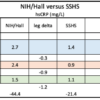
The chart on the left was published October 27, 2020 in MedRxiv: as Investigating hsCRP as a clinical inflammation marker for human Bisphenol A food contamination offers protocol suggestions for conducting replicable, causal dietary intervention studies (doi: https://doi.org/10.1101/2020.10.25.20212282). By contrast, the chart on the right, from September 2021 mass spec data, revealed scant evidence of […]