NOTE: This is an edited version of Appendix 3 of the “Revised Stealth Syndromes Study Protocol as approved by the University of California San Francisco Medical School Committee on Human Research.
Plastic chemicals contaminate our food because plastic isn’t just plastic. Instead, what we see as plastic is a mixture of one or more polymers along with a variety of chemical plasticizers and additives. These compounds are used to create polymers, or added to other polymers to provide desired characteristics such as color, strength, resistance to light, rigidity, flexibility and other qualities of the finished plastic.
This ad-free article is made possible by the financial support of the
Center for Research on Environmental Chemicals in Humans: a 501(c)(3) non-profit.
Please consider making a tax-deductible donation for continued biomedical research.
BPA & phthalates (and various other chemicas) migrate, leach and flake from plastics
BPA, phthalates, and other chemicals can migrate from, leach out of, and flake off of plastics primarily because they are not chemically bound to the polymers that make up the core of a plastic.
It’s important to understand that any specific plastic such as:
- Polycarbonate, (which is actually made by polymerizing BPA)
- Polyethelene
- Polyethelene terephthalate (PET)
is made from many different components including mixtures of different polymers.
This article from the Encyclopedia Britannica explains how various additives to polyethylene change its physical characteristics.
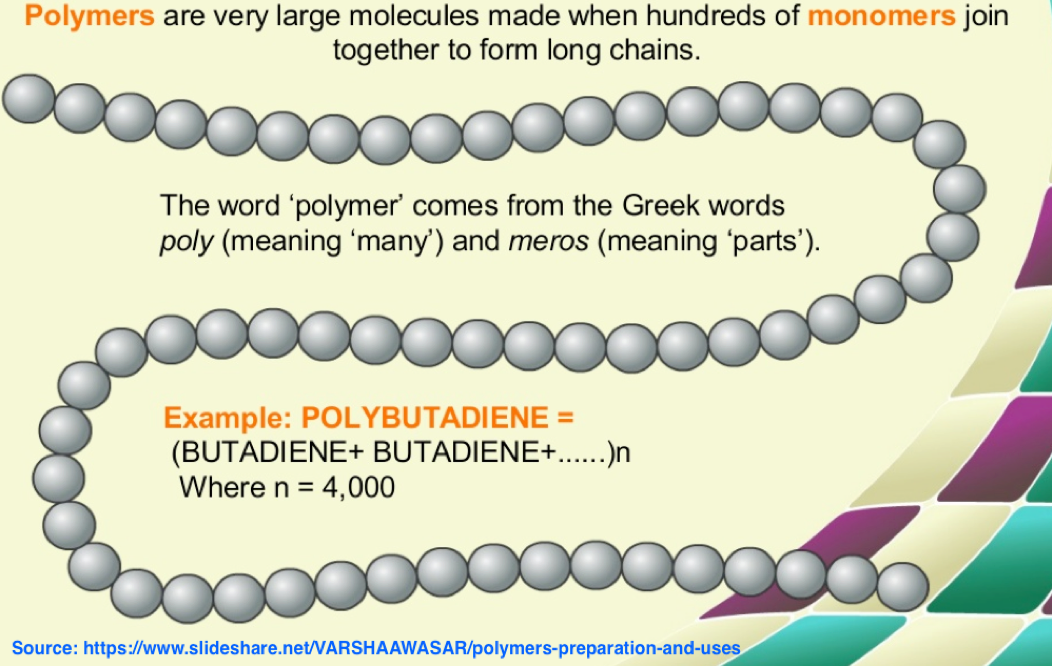
Polymers are a lot like cement and concrete.
In some ways, polymers are like dry cement.
Water can be added, and a hard substance results when it cures. Aggregates like sand and stone are mixed in to provide strength. Steel rebar is added for flexibility and tensile strength. Polymer fibers offer flexibility. Dyes are added for color.
An exothermic (heat-releasing) chemical reaction takes place when water is added to cement. But the sand, stones and re-bar are not part of the chemical reaction.
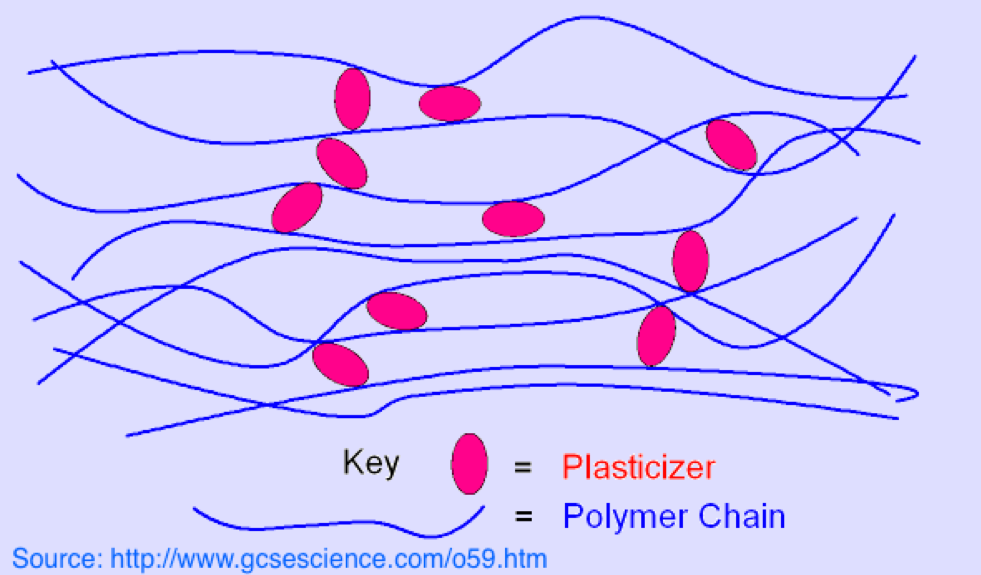
Just like plasticizers added to plastics, the additives to cement and not covalently bound to the concrete. “Covalent” bonding is the strongest form of binding atoms into a compound. Covalent happens when atoms in a compound share electrons.
Instead, those additives — whether to concrete or a plastic — are physically confined when the mixture cures, but can separate under many circumstances.
Rebar, stones and even sand are massive compared with the tiny sizes of the plasticizer and additive molecules that give polymer chains the desired characteristics.
Because of this, additives can migrate among the polymer chains and — when they reach the surface of the plastic — can easily leach or simply fall out of the plastic like pebbles from old concrete.
What helps additives escape from the plastic?
Additives escape from plastic and enter the food chain or directly into people’s bodies through a combination of actions that include:
Mechanical- Scraping, friction, bending, stretching, twisting or compressing plastic promotes microfractures that speed additive particles toward a surface. Friction between plastics or other components can create fine dust particles which accelerate the release of additives.
Mechanical stressors are particularly applicable to conveyor belts, squeezable condiment containers, plastic utensils in contact with plastic bowls or paper plates (most of which have a plastic coating), plastic gloves, and plastic beverage containers.
Children frequently treat their plastic toys and other objects with less than tender loving care.
Light – Degrades plastic and accelerates the release of plastic additives.
Chemical action – Acidic foods and beverages can react with a variety of additives because those are not chemically bound (covalently) to the polymers. Some polymers may also be subject to reacting with acids or other food and beverage compounds.
Lipophilia – Promotes absorption of chemicals in oil based foods. Studies show that people can absorb BPA from touching thermal paper cash receipts. What’s more, those who use hand moisturizers absorb far more BPA because of the increased lipids from the hand cream.
Heat – Adds energy to the migration process, speeds up chemical reactions that can loosen bonds.
Trade secrets leave all plastics under suspicion
The plastics business is insanely competitive.
Manufacturers are constantly developing new formulations to compete. As a result, the precise polymers and additives are trade secrets. Because of this, a manufactured plastic can be called “polyethelene” even though it has numerous other chemicals and polymers.
Manufacturers are not required to divulge the components of their plastics.
Further, no government regulations exist to require the testing of plastics for health or other safety reasons.
Realistically, a desire for reducing contamination risk means that all plastics involved with food and drink must be considered “guilty” and avoided.