NOTE: This is a summary of the current amended protocol.
The full study, including revised protocol and the expanded nutritional and food selection process as approved by the University of San Francisco Medical School Committee on Human Research can be accessed at this link (pdf).
This ad-free article is made possible by the financial support of the
Center for Research on Environmental Chemicals in Humans: a 501(c)(3) non-profit.
Please consider making a tax-deductible donation for continued biomedical research.
A previously approved (UCSF-IRB/CHR) version that includes numerous footnoted references describing the scientific and health needs for the study can be found at this link.
Internal web footnotes may not work as well in this web summary version as they do in the full 66-page pdf. However, exterior links to published papers and other sources are operational.
Revised Protocol: UCSF IRB/CHR Number: 15-17703
Approved by IRB/CHR
Co-Principal Investigators:
W. Lewis Perdue, Dr. Victor I. Reus MD
Study Title: Clinical blood profile assays as biomarkers to directly assess potential health effects resulting from the controlled elimination of suspected dietary and environmental chemical toxins.
This study is the first of its kind to go beyond simply measuring serum and urine concentrations of the subject chemicals. Instead, this study will measure clinically useful outcomes using widely accepted diagnostic tests available at most medical facilities.
The investigators of this study recommend a revised protocol to increase reproducibility and decrease costs.
This revision is based on 2-1/2 years of intensive research that has determined that the original protocol would be impractical, too expensive for allocated resources, and could not be replicated by other investigators.
These revisions did not result from a single incident of sudden enlightenment. Instead, they are the result of a steady accumulation of insight built upon the closer scrutiny of previously published diet intervention studies. Underpinning that was the accumulated discovery of confounding basic science factors never before considered in these previous studies. which rendered them irreproducible.
Chief among the non-replicability of is the fact that the food supply chain is ubiquitously contaminated with widely varying and uncontrolled levels of Bisphenol A, phthalates and other environmental chemicals.
The investigators believe they have developed a reliable method of preparing and sourcing foods that will allow consistent results across other investigations.
NOTE: This revised protocol is followed by four appendices containing supplemental material excerpted from our internal reproducibility problem-solving working paper that offers greater perspective on the rationale and feasibility of the revised protocol.
Appendix 1 – Reproducibility
Appendix 2 – Detailed parameters of intervention diet selections
Appendix 3 – How does the food chain get contaminated?
Appendix 4 – Additional references: Non-food contamination sources
ORIGINAL PROTOCOL AS APPROVED BY UCSF IRB/CHR
Expense and Practicality Issues
As we proceeded to implement the original protocol, we were continually confronted with a self-reinforcing cascade of confounding factors.
Among these confounding factors we discovered were:
- Extreme variations among published studies that measured BPA and phthalate concentrations in many common food items.[i],[ii],[iii],[iv],[v],[vi],[vii]
- Evidence of inherent contamination in production and processing and not solely from food contact materials.[viii],[ix],[x],[xi],[xii],[xiii],[xiv]
- Ubiquitous contamination of all commonly available foods. See Appendix 3.
- The necessity to test every food ingredient for nutrition and contamination levels.
- Substantial controls that needed to be imposed on non-food contamination sources.
- Lengthy testing regime a barrier to subject compliance.
Need to Increase Reproducibility
- Expanded data capture and transparency
- More demanding and selective food sourcing standards
- Minimization of non-food contamination sources
- More rigorous controls over experimental apparatus
NEW PROPOSED PROTOCOL:
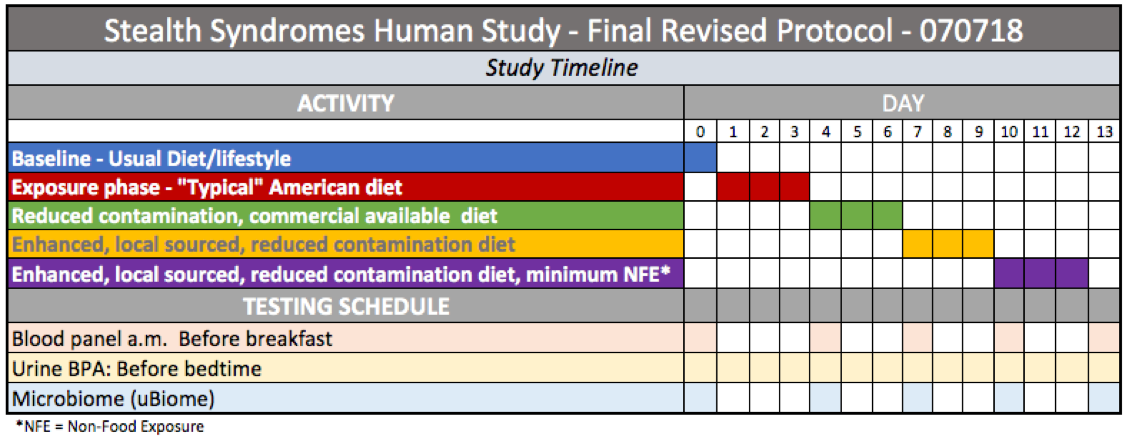
Protocol Phases
Baseline
Testing establishes the study subject’s pre-trial parameters. This also allows a comparison with NHANES data to see how test subject levels compare with the national median.
PHASE 1: Exposure, Typical American Diet
During this three-day period, study subjects will consume a diet designed to emulate a “typical” American diet.
To create that diet, investigators will rely, in part, upon the U.S. Department of Agriculture’s 2016 study: Americans’ Eating Patterns and Time Spent on Food: The 2014 Eating & Health Module Data[xv]. A more recent edition will be relied upon if available.
The search will begin in the frozen foods section of a supermarket. Ultimately, the retailer may be Walmart because they are located in all American states making specific brands easily accessible to other investigators.
Our assumption for this strategy is that a branded frozen food is likely to be consistent in content, nutrition and contaminant exposure regardless of the location of purchase. This is not a certainty because processing may have taken place at different facilities depending upon distribution and location of sale. We will make an effort to determine the processing facility for all goods.
Because data from numerous sources indicate that chicken, beef and pork are the most popular meats, we will reflect this by using each for one of the evening dinner menus during the 3-day exposure phase.
Because people in all demographics increasingly choose convenient foods that save time, we will choose complete meals with an emphasis on being as nutritionally balanced as possible — meat + vegetable (preference on leafy green or cruciform) + carbohydrate (pasta, potato etc.).
In cases where a suitable complete meal is not available, we will select multiple items and combine as a meal. Items will be prepared as indicated on their labels. This will, inevitably mean microwaving frozen foods in plastic containers or in paper containers with plastic liners.
To best reflect “typical American” meal and to assure widespread availability and reproducibility, we will select best-selling brands.
PHASE 2 – Reduced contamination, commercial
Once a menu has been established for the typical exposure phase, the components of this diet phase will be structured to emulate the typical exposure phase meals as closely as possible using less-contaminated food choices.
This phase, which has been missing from all other diet intervention studies of BPA and phthalates, offers study participants a “standard dose” of BPA and phthalates. This phase is intended not only to assess the effects of a typical American diet, but also to “standardize” food-exposure levels for each participant.
Standardized food exposure levels, combined with the final phase (minimum non-food exposure – NFE) may be useful in establishing estimates of overall individual NFE.
Foods and beverages will be obtained from Organic Certified sources, selected for minimum processing and plastic food contact materials. Currently, Whole Foods is the only national chain that prohibits biosolid use in its foods and may be a primary source because its availability facilitates reproducibility by other investigators.
Other widely available sources will be sought, especially from among direct-shipping vendors who adhere to the enhanced organic rules developed by this study’s investigators.
- No plastic contact. Exceptions are not preferred, but may include BPA and phthalate-free nitrile gloves and tubing such as Tygon S3 B-44-3 Beverage Tubing or other manufacturer’s equivalent.
- Any plastic product used must be tested to assure manufacturer claims because studies have shown that some manufacturer claims are false.[xvi],[xvii],[xviii]
Preparation & cooking
The following are forbidden:
- Sous vide
- non-stick pans
- most cooking oils
- plastic utensils
- plastic prep bowls
- synthetic gloves
- plastic bags
- plastic wrap
- drip coffee makers
- Sodastream
- Keurig and other “pod” beverage makers
- Beverages in cans, plastic bottles or glass.
PHASE 3: Enhanced organic
This diet will work to provide identical menu items served in previous phases but will follow a set of guidelines developed by this study’s investigators for sourcing local food and beverage products. Those guidelines eliminate many sources of endocrine disruptors — such as recycled wastewater irrigation — that are allowable under USDA organic regulations. Adherence to these rules should facilitate reproducibility.
The guiding principals are summarized below. More details are available in Appendix 2 of this document.
- Commercially processed foods are unacceptable.
- All food will be obtained directly from local Certified USDA Organic sources whose premises have been voluntarily inspected for compliance with those and the following enhanced requirements.
- Do not consume any ingredient whose composition cannot be traced to, and inspected at its origin.
- No recycled wastewater is allowed in irrigation or other on-premises uses.
- No biosolids are allowed in irrigation or other on-premises uses.
- No food contact with plastics or recycled paper or cardboard. Minimum or incidental contact may be approved depending upon the plastic composition.
- Irrigation water must not be transported via plastic pipes. Drip irrigation is discouraged, but if used must not contact edible surfaces.
- Harvest and food transport must be in metal containers.
PHASE 4 – Enhanced, local sourced, reduced contamination diet, minimum Non-Food Exposure
The unknown — and possibly unknowable — variabilities in this class of exposures offers the greatest set of hazards to reproducibility.
Numerous non-food sources of BPA are present in the environment and likely pose significant “background” contamination which will vary depending upon the test subjects’ lifestyles and environments.
This is particularly critical because the major health effects being measured in this study; estrogen/testosterone disruptions, inflammation, and metabolic events such as insulin resistance and lipid imbalance, can also be affected by a wide variety of chemicals other than BPA and phthalates.
This is why this final phase of the protocol has been added to reduce non-food exposures of all harmful chemicals as much as possible.
In addition, the personal environments of participants (personal care products, medications, etc.) must be noted for the record and reduced in a manner that can be illuminated by comparison with measured BPA, phthalate, and microbiome levels and indicators.
The path to a revised protocol: Confounding factors lead to greater complexity and expense
This study’s investigators, from the beginning, recognized that basic nutritional levels — protein, fat, and sugar/carbohydrates would need to be the same at each level of the study. While unnecessary in previous dietary intervention studies that measured only BPA levels, the present study also measures health effects which can be affected by nutritional composition.
The widespread variance in BPA concentrations in source foods led to the realization that reproducibility of this study would require testing of all source food for BPA concentrations. Otherwise, any record of increase or decrease in subject BPA concentrations would be invalid.
That recognition led to the conclusion that a reproducible investigation required the establishment of specific baseline food concentrations — leading to accurate dose levels of BPA consumed.
Further investigation led to published papers that indicated interactions between folic acid and BPA levels[xix]. That realization made it necessary to eliminate foods which would be concerned considered methyl contributors including genistin, and soy products among others.
This was further confounded by the revelation that BPA interacts with a key blood panel indicator, Prostate-Specific Antigen (PSA)[xx]. A clinical study included as part of this paper indicates that BPA may suppress serum levels of PSA in men under 65 years of age.
The potential confounding factors regarding PSA and folic acid led us to the realization that, in addition to assuring that the intervention diet nutritional content was equal in macronutrients to the baseline, we would also need nutritional analysis of micronutrients as well as every food item consumed. This compounded the complexity of the investigation as well as the costs.
Investigators’ further research indicated that the American food chain was so contaminated at every level by BPA and phthalates. The resulting food sourcing and testing demands increased the costs of the study far beyond the previous budget and would require extraordinary — and expensive — food production and processing methods to be implemented at the farm level and continuing in a highly controlled regime through processing, and preparation for table consumption.
This would require financial and personnel resources far beyond those originally anticipated.
The original study had, as its expressed outcomes to:
- Determine direct health effects: Determine if reducing BPA and phthalate levels correlated significantly with health effects that could be measured using well-accepted diagnostic blood panel elements, and
- Clinical usefulness: Offer consumers and their health professionals practical, evidence-based dietary intervention steps to improve personal health.
The ubiquitous, and inconsistent contamination of food, along with the limited availability of minimally, contaminated foods made those two goals impossible without testing every sample of food destined for the consumer’s mouth. This was obviously impractical.
This situation led us to a deeper re-examination of the handful of previous dietary interventions aimed at reducing bisphenol A concentrations in a test population. Not surprisingly, none of those previous could be replicated. Only one of those studies devoted significant attention to the reasons for non-reproducibility.
A more detailed discussion of those previous studies and this new protocol’s replication improvements is presented at the end of this document.
The evolution of this new protocol
Taking into account the results of previous published studies that indicated the ubiquitous presence of BPA and/or phthalates in every type of readily available food, the investigators sought to identify contamination sources, categorize them by food type and develop a simple, cost-effective method for selecting minimally contaminated foods.
The limited number of foods available for the intervention and variability of contamination meant that the granularity of measuring health effects by the stepwise reduction of seven different categories of food and beverages would yield little in useful data.
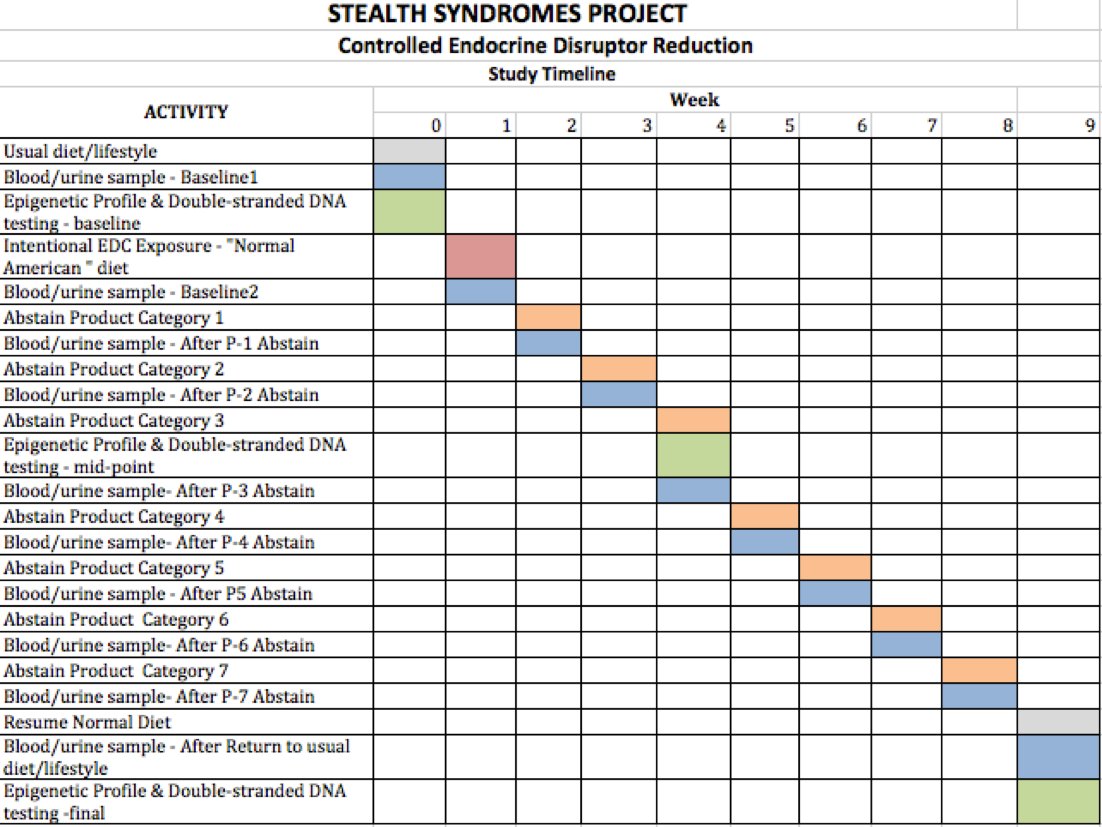
The original protocol offered a finer “granularity” of data because it called for nine test phases, each one week long which involved the step-wise elimination of seven contaminated product categories.
The theory was that, if BPA levels in test subjects dropped after eliminating a category, then that category was probably the cause of a certain level of contamination as indicated by blood and serum levels.
Cost constraints — primarily associated with testing costs — make the original protocol’s granularity goal impractical. For that reason, we propose four phases of three days each.
In addition, using week-long intervention periods for each category required extensive, and expensive meal preparation expense, increased risk of inadvertent contamination from non-food sources and — most likely — decreased compliance by test subjects.
The numerous additional phases and longer duration in the original protocol would not only increase the cost of testing, but would also increase food expense, impose unacceptable demands on test subjects, and place additional efforts on the reduction of non-food exposure sources.
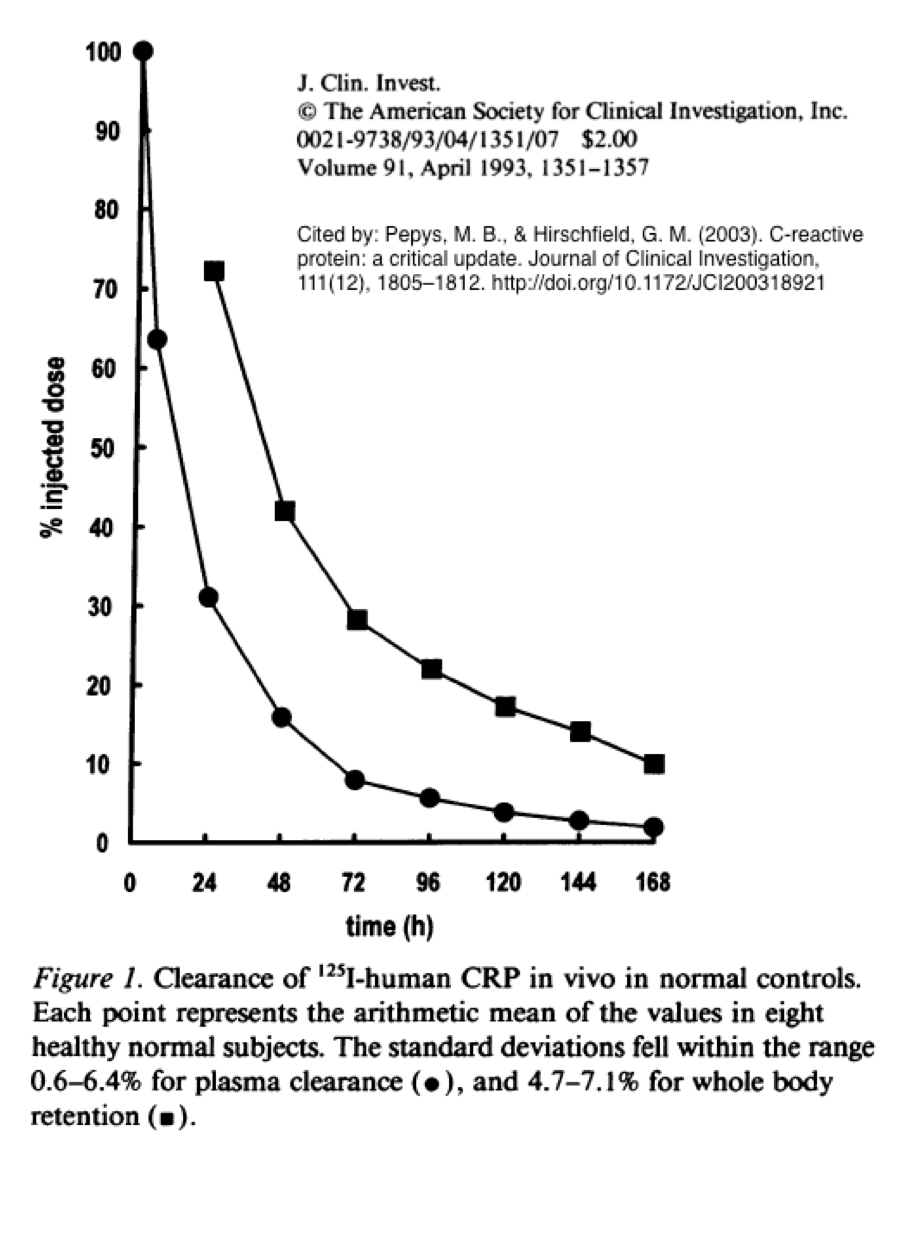
Adjusting protocol phases to accommodate CRP pharmacokinetics
While BPA levels rise and drop quickly within a day, our key health indicator — C-Reactive Protein (CRP) requires a different protocol.
Given CRP’s rapid response to initial stimulus, 3-day phases should allow adequate time for accurate measurements even though more data need to be located regarding the concentration of BPA needed to stimulate an adequate CRP synthesis response.
According to Pepys and Hirschfield (2003)[xxi], CRP is very responsive to inflammatory compounds: “De novo hepatic synthesis starts very rapidly after a single stimulus, serum concentrations rising above 5 mg/l by about 6 hours and peaking around 48 hours. The plasma half-life of CRP is about 19 hours ….”
Proposed blood panel markers
It would be desirable, if the pharmacokinetics and costs fit the protocol schedule and budget, to add additional data including a panel of inflammation markers including IL-1β, IL-6, IL-10, and TNF-α and an oxidative stress panel.
While those biomarkers include indications of conditions related to Type 2 diabetes and carcinomas, it would also be desirable to include measurements of leptin, and appropriate members of the VEGF and FGF families.
Finally, given that Bisphenol A is an estrogenic compound and phthalates are anti-androgenic, a measurement of the androgen/estrogen ratio for all test participants would be appropriate because Bisphenol A is associated with hormone related cancers including breast and prostate.[xxii]
PSAs tests for men should be included as well since a study published in 2014 found exposure to bisphenol A correlates with early-onset prostate cancer[xxiii] and that BPA exposure may lower serum PSA levels, thus complicating diagnosis. See also: Role of diet in prostate cancer: the epigenetic link[xxiv].
Regardless of which tests may be appropriate and affordable (beyond CRP) this revised protocol will save significant time and cost.
New testing regime: Epigenetic profile, BPA serum dropped, gut microbiome added
The epigenetic profiling will be eliminated as a budget move unless a suitable co-investigator or testing source can be located and additional funds allocated.
One potentially useful result from the original protocol was investigating the health effects of reduced BPA and phthalate concentrations on a diagnostic blood panel and comparing the results with BPA serum and urine concentrations. However, The BPA serum testing is included in the revised protocol but may also need to be eliminated for budgetary reasons.
As previously noted in the protocol, some controversy has lingered over the presence of free BPA in serum. Many investigators — primarily traditional toxicologists — have insisted that free serum BPA does not exist and that all BPA is absorbed by digestive tract mechanisms, metabolized by the liver and excreted.
The controversy exists because alternate pathways for dietary BPA to enter the bloodstream have been proposed including transdermal absorption by buccal tissues including the dental gums.
Samples for BPA serum testing. if retained in the testing schedule, would be obtained at the same time as the blood panel.
Gut Microbiome added
One additional testing method proposed for this revised protocol includes gut microbiome measurements.
The science of gut microbiome measurements and the ability to sample and analyze the results conveniently and inexpensively has advanced greatly in the past three years.
In addition, development of clinical diagnostic techniques have also made great strides.
Therefore, the relationships among these methods: diagnostic blood panel, urine, and gut microbiome, offer the possibility of yielding novel and significant health effects of BPA and phthalates on gut biome composition.
Data gathered from the use of these methods could be the subject of a separate paper.
Economics: Testing for BPA Only
In addition, testing will be restricted to BPA. Phthalates and other endocrine disruptors are frequently detected together in the same samples. In this case, BPA becomes a “marker” for chemicals having similar health effects.
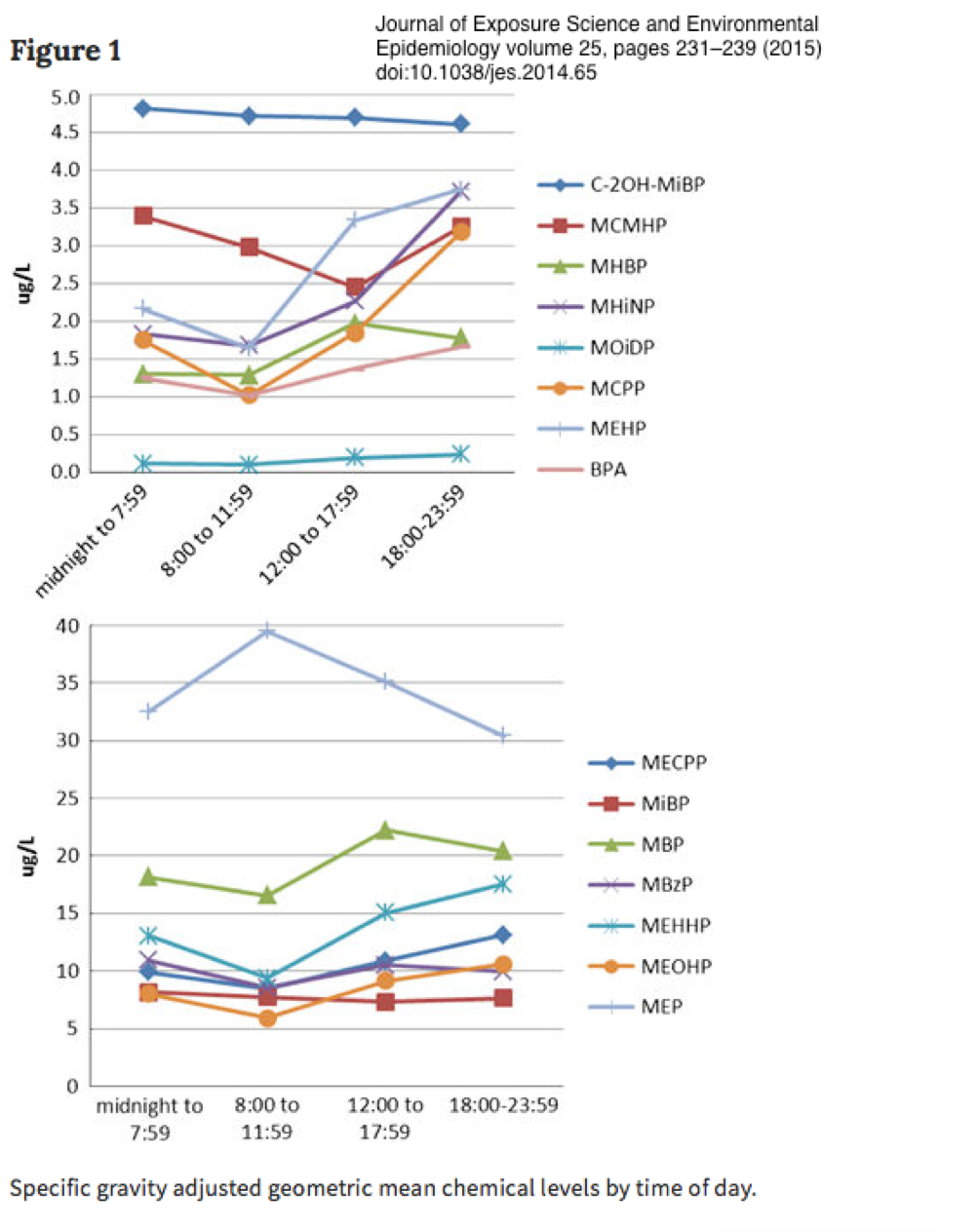
Both Bisphenol A and phthalates are rapidly metabolized[xxv] and decline rapidly within 8 hours, which makes a three-day cycle appropriate for testing.
While one important study indicated that BPA had a longer half life, the methodology of that study indicated that a slower decline could be due to release from adipose stores or continual contamination from non-food sources[xxvi]. Significantly, the three-day period for each diet segment, and daily urine BPA testing, should accommodate data for both studies.
Phthalate testing would be valuable, but is prohibitively expensive given that its ubiquitous environmental presence requires specialized and extensive laboratory precautions which vastly reduces the number of qualified testing facilities.
Data also indicate[xxvii] that Bisphenol A will serve as an appropriate marker for phthalates because urinary concentrations of both decrease together with very similar data curves[xxviii] for most (but not all) phthalates. In addition, phthalates, parabens, bisphenol A and triclosan have shown similar concentration correlations[xxix].
Focus on reproducibility as a function of usefulness, practicality, cost, and transparency.
Indeed, the inability of other investigators to reproduce previous dietary studies on this subject (see discussion below) can be laid at the feet of the numerous confounding factors the current researchers have thoroughly investigated in an effort to source appropriate food and beverage for the study.
Those previous dietary studies attempted only to correlate test subject urine concentrations of BPA metabolites. Our study, on the other hand, intends to extend the knowledge gained in those by correlating BPA and phthalate levels to direct human health effects as determined by accepted laboratory blood tests and other indicators.
Lessons from the Non-Replicability of Previous Studies
There have been five dietary intervention studies regarding BPA, phthalates, or both.
Three are not relevant because of fundamental flaws:
- One involved members of a single family, all of whom had very low baseline BPA levels (some below LOD) because of an existing habit of avoiding plastics: Life without plastic: A family experiment and biomonitoring study.[xxx]
- A second recruited a cohort of guests at a spiritual retreat: Influence of a five-day vegetarian diet on urinary levels of antibiotics and phthalate metabolites: a pilot study with “Temple Stay” participants.[xxxi]
- The third was self-administered by high-school students given a list of foods to avoid: An engaged research study to assess the effect of a ‘real-world’ dietary intervention on urinary bisphenol A (BPA) levels in teenagers.[xxxii]
Of these, only two are relevant:
- The first broke new ground as the first of its kind with solid scientific underpinnings: Food Packaging and Bisphenol A and Bis(2-Ethyhexyl) Phthalate Exposure: Findings from a Dietary Intervention.[xxxiii]
- The second attempted to replicate the first study. And found that some of the participants had higher levels of BPA and phthalates after intervention: Unexpected results in a randomized dietary trial to reduce phthalate and bisphenol A exposures[xxxiv].
According to Sathyanarayana et al. (2013)[xxxv], “tested food ingredients from the same suppliers but were unable to test the exact same food ingredients used in the intervention because testing was performed 3 weeks after the study was completed.”
In the absence of data, the investigators reasoned that the unexpected increases came from far more than food contact material and reasoned that “the food supply is systematically contaminated….”
Finally, they concluded “Federal or industry wide regulation aimed at reducing phthalate and BPA concentrations in foods may be the only effective mechanism to ensure the food supply is safe from contamination.”
See Appendix 3 for a discussion of food contamination sources which occur prior to contact with plastic packaging materials.
Learning from the replication efforts of the past
This revised protocol has been expanded to address the confounding factors discovered above along with numerous other serious problems discovered by investigators over the past 2 1/2 years.
Our research has revealed numerous contamination source and factors that afflict every level of the food chain from production to preparation for consumption.
Therefore, the lessons of Sathyanarayana et al. (2013) comprise the first of several novel recommendations/innovations to improve replicability for food intervention studies
Current investigators took careful note of the following conclusions of Sathyanarayana et al. (2013) who had worked “to develop a controlled dietary replacement focused on fresh, organic, and local foods without the use of plastics.
- “We conclude that currently accepted methods to reduce phthalate and BPA exposures (both dietary replacement and written recommendations) may not lead to anticipated changes in urinary phthalate and BPA concentrations.”
- “Our study team undertook several measures to ensure that our dietary replacement would consist of fresh, local, organic food prepared, stored, and transported without plastics. For example, the caterer called local farms and asked that fresh foods be delivered in wood crates instead of plastic cartons.
- “All dairy was delivered in glass (milk/cream) or paper except for one delivery of butter in plastic. In the kitchen, the cooks prepared dishes without the use of plastic utensils, appliances, or storage containers.
- “Families were instructed to eat using ceramic dishes and metal utensils. We provided glass containers for food storage and transport. Despite these measures, DEHP metabolite concentrations increased significantly ….”
And, yet, those efforts were not enough.
We therefore conducted a thorough investigation to discover and circumvent every possible contamination in the food chain.
Reproducibility depends on the availability of the exact same foods, prepared the exact same way
Creating a practical, useful study which could be economically replicated must focus on foods which are widely available nationwide. As much as the investigators would like to conduct the study with the minimum-possible contamination, the “enhanced organic” sourcing would make it both expensive and impossible to replicate.
This is because replication requires total data transparency and that requires (among other things) the ability to source materials used in the original study.
Previous dietary studies of BPA and related endocrine disruptors have not made that information available.
Those studies have given instructions to food preparers to use only “fresh” ingredients and to avoid any plastic contact. However, specific food sources and the details of their production are not available. Neither are specific cooking protocols or the identities of the utensils, pots, pans, cleaning regimen (surfactants an issue), recipes, ingredients (processing & methyl contributors an issue).
In addition, no details are available on non-food-contamination variables, environmental conditions or precautions regarding the avoidance of those.
Data regarding those conditions cannot account for the wide variability of contamination in the growing, processing, and sourcing stages. As a result, instructions in previous dietary studies account only for contamination transfer from food packaging materials and preparation.
No studies have been found that parse the growing, production, and processing contamination from that of food contact materials and preparation.
This study cannot afford to measure all of those disparate contributions directly, but will attempt to control for that lack of information and facilitate replication using the guiding principles for enhanced organic, previously described above.
Obtaining food only from sources with nationwide availability and will make that full data available.
Treat the kitchen as a laboratory and pots, pans & utensils as lab equipment.
Along with enabling replication by controlling for the systematic contamination of the food supply, we will present detailed methods and procedures, preparation, apparatus standardization, cleaning and maintenance.
All of those previous details have been omitted from all previous studies.
The precise manufacturer, model, and size of pots, pans, dishes should be disclosed as well as food preparation utensils, and appliances. The source vendor should be noted.
To minimize incidental contamination, pots, pans, and their handles should be glass and/or stainless steel and un-coated (no Teflon or other non-stick treatments).
Cooking, serving and eating utensils should be stainless steel. Wooden utensils may be used for scraping if needed.
Glass must be used for all serving items including plates, bowls, cups, and drinking glasses. This practice avoids the possibility of incidental contamination from unknown substances leaching from glazes.
Effort must be made to assure that the lowest-priced suitable items are used and that exact duplicates are widely and easily available to encourage replication.
Automatic dishwashers may be used. Appropriate residue-free cleaning agents such as one of the Extran products from Millipore/Sigma, or those from Alconox may be used.
Several cycles using the detergent should be run to remove any residues present from commercial household products. The number of those cycles should be noted along with whether the input water to the dishwasher is filtered and whether it is fed by plastic pipes. The type of plastic should be noted.
Carbon filtered water is desirable, but the volume required is impractical and too expensive for most research efforts.
Cleaned items must be followed by a rinse in properly carbon-filtered water to remove any remaining residues including those left by the unfiltered water utilized by the home dishwasher. No plastic sponges or polymer-based towels or cloths may be used.
After rinse, items should be air-dried on a stainless steel rack.
They should not be dried with a towel. Synthetic fabrics and even those from natural fibers are frequently contaminated during manufacturing.[xxxvi]
Additional contamination can come from chemicals used in laundry detergents, softeners and dryer sheets that contain phthalates used as carriers for fragrances.
In addition, home dryer drums may be coated with stubborn layers of contaminants from drying fabric contaminated by detergents and from transfer from designs imprinted on tee-shirts and other clothing. Most inks used to print the designs on fabric are phthalate-based.
Recipes are laboratory procedures
Cooking is a chemistry procedure which must be described — and adhered to — by cook/chemists.
Ingredients are reagents and must be obtained from specified, trusted sources, measured precisely, and added at the proper time at the proper concentrations.
Recipes are experimental procedures that must be measured and timed precisely.
All of those details are analogous to laboratory data provided by in non-dietary studies which provide the specific names of reagents and apparatus along with formulae and other information necessary for experiment replication.
All of those details will available with other study data and supplemental material.
And while expense prevents this study from testing the precise contamination concentrations or measure nutritional profiles, the use of the same materials from the same sources offers an opportunity to increase the likelihood of successful replication.
Reproducibility: Reducing non-food exposures (NFEs)
Sathyanarayana et al. (2013) posited that substantial non-food exposures such as those with personal care products could mask BPA and phthalate reductions from dietary intervention.[xxxvii]
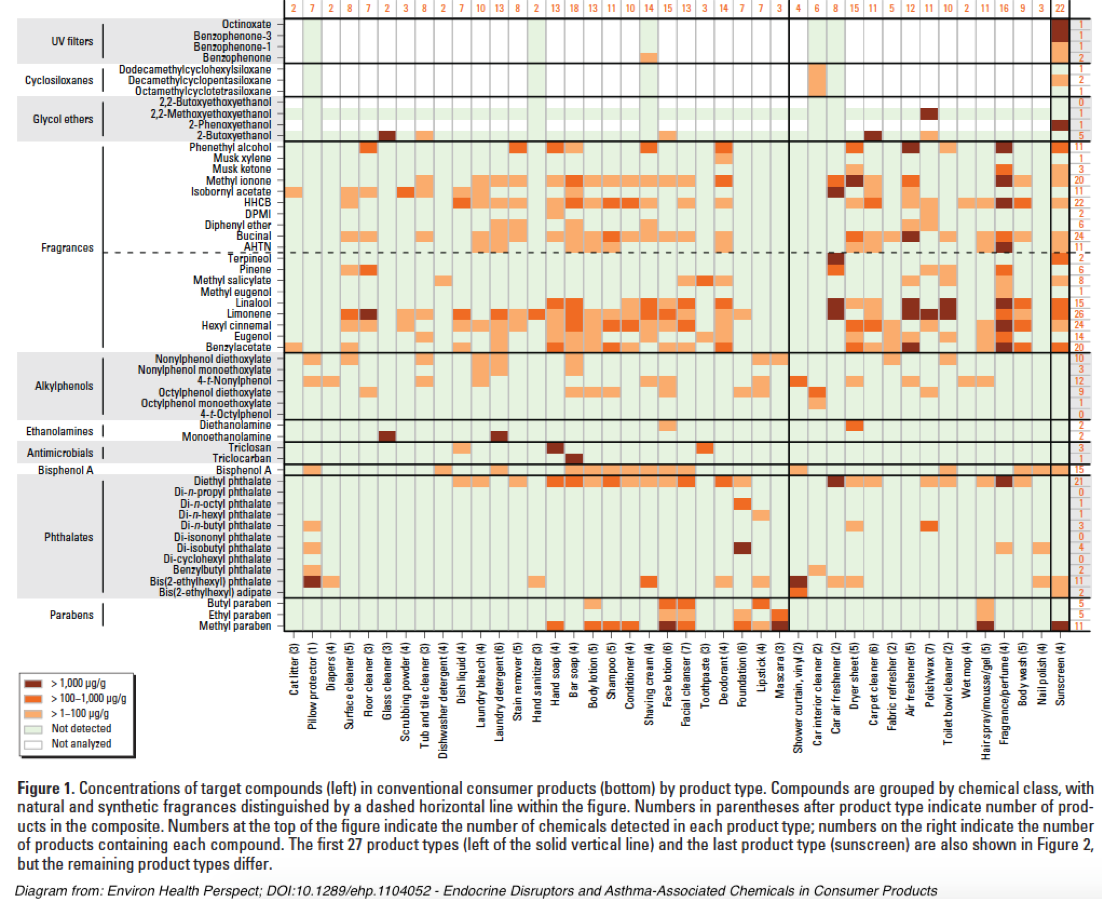
However, a quick picture of the complexity of NFEs can be illustrated by this diagram from Endocrine Disruptors and Asthma-Associated Chemicals in Consumer Products[xxxviii]:
An extensive discussion of NFE contamination sources may be accessed in Appendix 4.
Triaging NFE elimination
Given the impossibility of avoiding BPA, phthalates, and other harmful chemicals in the environment, reproducibility of studies must focus on the practical reduction of known exposures reducing those exposures that are:
- most easily identified and,
- practical to control,
- likely to produce maximum reduction via minimum study subject effort, and
- can be adequately documented and replicated.
An ideal scenario would be to conduct trials in a well-defined residential/dormitory setting where all study subjects experience near-identical environmental conditions for the entire term of he study.
However, expense and practicality dictate that many studies must engage subjects who will modify their diets while living in their own homes as well as engaged in multiple additional sites for work, school, shopping, and recreation.
Because of those practical limitations investigators should — to the best of their ability — thoroughly document the environmental circumstances under which a dietary intervention is conducted. This should include questionnaires about known exposure sources for their:
- External environment (home, work, school, recreation including floor surfaces) and,
- Personal environment (pharmaceuticals, personal care products, appliances, fabrics for clothing and bedding, presence of plastic dental and other implants and prosthetics, etc.).
Participants with excessive and/or uncontrollable external environmental exposures will be excluded.
Study subjects should then be counseled on contamination sources and given a checklist of specific controllable sources to avoid during the trial.
Investigators should schedule frequent reminders and follow-ups with each participant to assess and assure the best possible compliance.
As a potential starting point for assessing personal environmental exposures, investigators will consider the EDC Footprint Calculator developed by the Pennsylvania State University Extension Service. While this Excel version was designed to offer an indication of contamination products found in municipal wastewater and is not all-inclusive, it could be modified and updated to better serve the purposes of this study, or future efforts.
Personal environment controls
As the above chart from “Endocrine Disruptors and Asthma-Associated Chemicals in Consumer Products” illustrates, most personal care products contain relevant chemical contaminants that can potentially confound this study’s chosen health effects markers.
Select three-day reduction actions for personal environment:
NOTE: To make these reductions possible and assure the greatest possibilities for reproducibility, study subjects must be healthy, non-smoking adults without recent dental work or invasive medical procedures. Male subjects should not have body fat more than 32% for women and 25% for men. Subjects should not be taking any medicines or dietary supplements other than low-dose or 325 mg aspirin.
- Use recommended toothpaste without Triclosan and reduced or zero-levels of contaminants.
- No mouthwash.
- Uncoated nylon fiber dental floss (no plastic strips).
- No hand creams or lotions.
- No perfumes or any other products containing fragrances.
- Use recommended shampoo with reduced or zero-levels of contaminants.
- Use recommended hand soap with reduced or zero-levels of contaminants.
- No cosmetics.
- No hair color treatment.
- No teeth whitener.
- No hand sanitizers.
- Use recommended deodorant.
- No contact lenses. Use of eyeglasses only.
- No contact with cash register receipts.
- No hair spray, gel or other treatment.
- No body wash.
- No sunscreen.
- No nail polish.
- No tee-shirts, sweatshirts, or other clothing with printed designs.
In addition, subjects must note synthetic composition of clothing and bedding items. That data may be valuable for investigating possible confounding factors.
External environment controls
In addition to food contamination issues, home, workplace, and public spaces also provide significant — but widely variable — exposure to humans to endocrine disruptors, CECs and, other environmental chemicals[xxxix].
- Do not use ink jet printers, gel rollerball pens, ballpoint pens.
- No use of volatile adhesives such as rubber cement or from spray cans.
- Use no household cleaners.
- No reusable, non-paper grocery/shopping bags with printed designs.
- No plastic wallets/purses/backpacks.
- Inform investigators if beds or pillows have plastic covers.
- No air fresheners.
- Avoid areas irrigated with recycled wastewater.
- Do not get into a vehicle until the interior has been thoroughly ventilated with outside air.
- Ride in vehicles with all windows open. Do not use an air freshener. Avoid new vehicles and those whose interiors have been recently cleaned commercially.
- Wear only those clothes that have been washed with phthalate-free laundry detergents.
- Unless a phthalate-free laundry detergent has been used previously, run two loads of towels, then dry.
- If fabric softeners have been used in the dryer, run two additional loads of towels after decontaminating washer and dry them in the dryer to reduce accumulated phthalate levels.
- Avoid bathing in homes whose water supply is delivered with plastic plumbing: PVC, Pex etc.
Dust particles
Household dust presents a significant confounding factor because it is the residual detritus from many environmental sources shown to contain BPA, phthalates, and other chemicals relevant to this study: Phthalates in Indoor Dust and Their Association with Building Characteristics[xl]. That study contains a home materials inventory that will be adapted for the study subject questionnaire. See also PM 2.5[xli].
Reduction actions for dust particles:
Frequent and thorough cleaning (before the start of phase 4) of all surfaces (floor, walls, ceilings) with a mop dampened with water only. No cleaning compounds which may introduce their own unique, unknown, and uncontrollable contaminants.
If the cleaning/dusting is performed by the test subject, then tasks should be performed wearing an N-95/p100 mask/filter. Significantly, masks of that particular quality do not filter all of the most hazardous particulates — those in the 2.5 micron (PM 2.5) and smaller size.
The PM 2.5 size class is most likely to reach and be retained by alveoli and thus have direct access to the bloodstream.
As a consequence, PM 2.5 is implicated in a wide range of serious health effects[xlii],[xliii],[xliv].
The type and age of the HVAC system should be noted as well as the type of filter used and the ducting materials (metal versus plastic).
Immediately after dusting, the HVAC filter should be changed and the system fan run continuously for four hours to collect as much dust as possible which has been stirred up from cleaning. A fresh filter should then be installed.
The slightly-used filter may be preserved for future use if handled gently and immediately inserted into a suitably-sized plastic bag. Investigators note the ironic necessity to use plastic to help protect against plastic.
Study subjects who already have a HEPA-class air purifier should report the make, model, capacity and whether it is portable or installed with the HVAC system. If portable, the location should be noted along with the distance from the HVAC air return.
More supplemental references may be found in Appendix 5.
[i] Fasano, E., Bono-Blay, F., Cirillo, T., Montuori, P., and Lacorte, S. 2012. Migration of phthalates, alkylphenols, bisphenol A and di(2-ethylhexyl)adipate from food packaging. Food Control 27(1): 132-138.
[ii] Serrano, S.E., Braun, J., Trasande, L., Dills, R., and Sathyanarayana, S. 2014. Phthalates and diet: a review of the food monitoring and epidemiology data. Environmental Health 13: 43.
[iii] Guart, A., Bono-Blay, F., Borrell, A., and Lacorte, S. 2011. Migration of plasticizers phthalates, bisphenol A and alkylphenols from plastic containers and evaluation of risk. Food Additives & Contaminants Part A: Chemistry, Analysis, Control, Exposure & Risk Assessment 25(5): 676-685.
[iv] Bhunia, K., Sablani, S.S., Tang, J., and Rasco, B. 2013. Migration of chemical compounds from packaging polymers during microwave, conventional heat treatment, and storage. Comprehensive Reviews in Food Science and Food Safety 12(5): 523-545.
[v] Bang, D.Y., Kyung, M., Kim, M.J., Jung, B.Y., Cho, M.C., Choi, S.M., Kim, Y.W., Lim, S.K., Lim, D.S., Won, A.J., Kwack, S.J., Lee, Y., Kim, H.S., and Lee, B.M. 2012. Human risk assessment of endocrine-disrupting chemicals derived from plastic food containers. Comprehensive Reviews in Food Science and Food Safety 11(5): 453-470.
[vi] Groh, K.J., Geuke, B., and Muncke, J. 2017. Food contact materials and gut health: Implications for toxicity assessment and relevance of high molecular weight migrants. Food and Chemical Toxicology 109(1): 1-18.
[vii] Bittner, G.D., Denison, M.S., Yang, C.Z., Stoner, M.A., and He, G. 2014. Chemicals having estrogenic activity can be released from some bisphenol a-free hard and clear, thermoplastic resins. Environmental Health 13: 103.
[viii] Schecter, A., Lorber, M., Guo, Y., Wu, Q., Yun, S.H., Kannan, K., Hommel, M., Imran, N., Hynan, L.S., Cheng, D., Colacino, J.A., and Birnbaum, L.S. 2013. Phthalate concentrations and dietary exposure from food purchased in New York State. Environmental Health Perspectives 121(4): 473-479.
[ix] Cariou, R., Larvor, F., Monteau, F., Marchand, P., Bichon, E., Dervilly-Pinel, G., Antignac, J-P., and Le Bizec, B. 2016. Measurement of phthalates diesters in food using gas chromatography-tandem mass spectrometry. Food Chemistry 196: 211-219.
[x] Van Holderbeke, M., Geerts, L., Vanermen, G., Servaes, K., Sioen, I., De Henauw, S., and Fierens, T. 2014. Determination of contamination pathways of phthalates in food products sold on the Belgian market. Environmental Research 134: 345-352.
[xi] Fasano, E., Bono-Blay, F., Cirillo, T., Montuori, P., and Lacorte, S. 2012. Migration of phthalates, alkylphenols, bisphenol A and di(2-ethylhexyl)adipate from food packaging. Food Control 27(1): 132-138.
[xii] Cirillo, T., Latini, G., Castaldi, M.A., Dipaola, L., Fasano, E., Esposito, F., Scognamiglio, G., Di Francesco, F., and Cobellis, L. 2015. Exposure to di-2-ethyhexyl phthalate, di-N-butyl phthalate and bisphenol A through infant formulas. Journal of Agricultural and Food Chemistry 63(12): 3303-3310.
[xiii] Fierens, T., Vanermen, G., Van Holderbeke, M., De Henauw, S., and Sioen, I. 2012. Effect of cooking at home on the levels of eight phthalates in foods. Food and Chemical Toxicology 50(12): 4428-4435.
[xiv] Ionas, A.C., Dirtu, A.C., Anthonissen, T., Neels, H., and Covaci, A. 2014. Downsides of the recycling process: Harmful organic chemicals in children’s toys. Environment International 65: 54-62.
[xv] Hamrick, K., and McClelland, K. 2016. Americans’ Eating Patterns and Time Spent on Food: The 2014 Eating & Health Module Data. Economic Information Bulletin No. (EIB-158); 51pp.
[xvi] Guart, A., Wagner, M., Mezquida, A., Lacorte, S., Oehlmann, J., and Borrell, A. 2013. Migration of plasticizers from TritanTM and polycarbonate bottles and toxicological evaluation. Food Chemistry 141(1): 373-380.
[xvii] Bittner, G.D., Yang, C.Z., and Stoner, M.A. 2014. Estrogenic chemicals often leach from BPA-free plastic products that are replacements for BPA-containing polycarbonate products. Environmental Health 13: 41.
[xviii] Bittner, G.D., Denison, M.S., Yang, C.Z., Stoner, M.A., and He, G. 2014. Chemicals having estrogenic activity can be released from some bisphenol a-free hard and clear, thermoplastic resins. Environmental Health 13: 103.
[xix] Dolinoy, D.C., Huang, D., and Jirtle, R.L. 2007. Maternal nutrient supplementation counteracts bisphenol A-induced DNA hypomethylation in early development. PNAS 104(32): 13056-13061.
[xx] Tarapore, P., Ying, J., Ouyang, B., Burke, B., Bracken, B., and Ho, S.M. 2014. Exposure to bisphenol A correlates with early-onset prostate cancer and promotes centrosome amplification and anchorage-independent growth in vitro. PLoS One 9(3): e90332.
[xxi] Pepys, M, B., and Hirschfield, G.M. 2003. C-reactive protein: A critical update. The Journal of Clinical Investigation 111(12): 1805-1812.
[xxii] Gao, H., Yang, B-J., Li, N., Feng, L-M., Shi, X-Y., Zhao, W-H., and Liu, S-J. 2015. Bisphenol A and Hormone-Associated Cancers: Current Progress and Perspectives. Medicine 94(1): e211.
[xxiii] Tarapore, P., Ying, J., Ouyang, B., Burke, B., Bracken, B., and Ho, S.M. 2014. Exposure to bisphenol A correlates with early-onset prostate cancer and promotes centrosome amplification and anchorage-independent growth in vitro. PLoS One 9(3): e90332.
[xxiv] Labbé, D.P., Zadra, G., Ebot, E.M., Mucci, L.A., Kantoff, P.W., Loda, M., and Brown, M. 2014. Role of diet in prostate cancer: the epigenetic link. Oncogene 34: 4683-4691.
[xxv] Fisher, M., Arbuckle, T.E., Mallick, R., LeBlanc, A., Hauser, R., Feeley, M., Koniecki, D., Ramsay, T., Provencher, G., Bérubé, R., and Walker, M. 2015. Bisphenol A and phthalate metabolite urinary concentrations: Daily and across pregnancy variability. Journal of Exposure Science & Environmental Epidemiology 25: 231-239.
[xxvi] Stahlhut, R.W., Welshons, W.V., and Swan, S.H. 2009. Bisphenol A data in NHANES suggests longer than expected half-life, substantial nonfood exposure, or both. Environmental Health Perspectives 117(5): 784-789.
[xxvii] Song, Y., Hauser, R., Hu, F.B., Franke, A.A., Liu, S., and Sun, Q. 2014. Urinary concentrations of bisphenol A and phthalate metabolites and weight change: a prospective investigation in US women. International Journal of Obesity 38(12): 1532-1537.
[xxviii] Fisher, M., Arbuckle, T.E., Mallick, R., LeBlanc, A., Hauser, R., Feeley, M., Koniecki, D., Ramsay, T., Provencher, G., Bérubé, R., and Walker, M. 2015. Bisphenol A and phthalate metabolite urinary concentrations: Daily and across pregnancy variability. Journal of Exposure Science & Environmental Epidemiology 25: 231-239.
[xxix] Larsson, K., Björklund, K.L., Palm, B., Wennberg, M., Kaj, L., Lindt, C.H., Jönsson, B.A.G., and Berglund, M. 2014. Exposure determinants of phthalates, parabens, bisphenol A and triclosan in Swedish mothers and their children. Environment International 73: 323-333.
[xxx] Hutter, K.P., Kundi, M., Hohenblum, P., Scharf, S., Shelton, J.F., Piegler, K., and Wallner, P. 2016. Life without plastic: A family experiment and biomonitoring study. Environmental Research 150: 639-644.
[xxxi] Ji, K., Lim Kho, Y., Park, Y., and Choi, K. 2010. Influence of a five-day vegetarian diet on urinary levels of antibiotics and phthalate metabolites: a pilot study with “Temple Stay” participants. Environmental Research 110(4): 375-382.
[xxxii] Galloway, T.S., Baglin, N., Lee, B.P., Kocur, A.L., Shepherd, M.H., Steele, A.M., BPA Schools Study Consortium, and Harries, L.W. 2018. An engaged research study to assess the effect of a “real-world” dietary intervention on urinary bisphenol A (BPA) levels in teenagers. BMJ Open 8: e018742.
[xxxiii] Rudel, R.A., Gray, J.M., Engel, C.L., Rawsthorne, T.W., Dodson, R.E., Ackerman, J.M., Rizzo, J., Nudelman, J.L., and Brody, J.G. 2011. Food packaging and Bisphenol A and Bis(2-Ethyhexyl) Phthalate exposure: Findings from a dietary intervention. Environmental Health Perspectives 119(7): 914-920.
[xxxiv] Sathyanarayana, S., Alcedo, G., Saelens, B.E., Zhou, C., Dills, R.L., Yu, J., and Lanphear, B. 2013. Unexpected results in a randomized dietary trial to reduce phthalate and bisphenol exposures. Journal of Exposure Science & Environmental Epidemiology 23: 378-384.
[xxxv] Sathyanarayana, S., Alcedo, G., Saelens, B.E., Zhou, C., Dills, R.L., Yu, J., and Lanphear, B. 2013. Unexpected results in a randomized dietary trial to reduce phthalate and bisphenol exposures. Journal of Exposure Science & Environmental Epidemiology 23: 378-384.
[xxxvi] Xue, J., Liu, W., and Kannan, K. 2017. Bisphenols, Benzophenones, and Bisphenol A Diglycidyl Ethers in Textiles and Infant Clothing. Environmental Science & Technology 51(9): 5279-5286.
[xxxvii] Sathyanarayana, S., Alcedo, G., Saelens, B.E., Zhou, C., Dills, R.L., Yu, J., and Lanphear, B. 2013. Unexpected results in a randomized dietary trial to reduce phthalate and bisphenol exposures. Journal of Exposure Science & Environmental Epidemiology 23: 378-384.
[xxxviii] Dodson, R.E., Nishioka, M., Standley, L.J., Perovich, L.J., Brody, J.G., and Rudel, R.A. 2012. Endocrine disruptors and asthma-associated chemicals in consumer products. Environmental Health Perspectives 120(7): 935-943.
[xxxix] Song, Y., Hauser, R., Hu, F.B., Franke, A.A., Liu, S., and Sun, Q. 2014. Urinary concentrations of bisphenol A and phthalate metabolites and weight change: a prospective investigation in US women. International Journal of Obesity 38(12): 1532-1537.
[xl] Bornehag, C-G., Lundgren, B., Weschler, C.J., Sigsgaard, T., Hagerhed-Engman, L, and Sundell, J. 2005. Pthalates in indoor dust and their association with building characteristics. Environmental Health Perspectives 113(10): 1399-1404.
[xli] Marshall, J. 2013. PM 2.5. PNAS 110(22): 8756.
[xlii] Schwartz, J., Laden, F., and Zanobetti, A. 2002. The concentration-response relation between PM(2.5) and daily deaths. Environmental Health Perspectives 110(10): 1025-1029.
[xliii] Pope III, C.A., Ezzati, M., Cannon, J.B., Allen, R.T., Jerrett, M., and Burnett, R.T. 2018. Mortality risk and PM2.5 air pollution in the USA: an analysis of a national prospective cohort. Air Quality, Atmosphere & Health 11(3): 245-252.
[xliv] Franklin, M., Zeka, A., and Schwartz, J. 2007. Association between PM2.5 and all-cause and specific-cause mortality in 27 US communities. Journal of Exposure Science & Environmental Epidemiology 17: 279-287.